Stainless steel corrosion is one of the worst nightmares of every architect, constructor, or engineer. Imagine you are building a bridge, wind turbine, or skyscraper and the steel-aluminum connection starts to corrode. We are talking here about safety issues and potential disasters! You absolutely want to avoid stainless steel corrosion and this article tells you how.
What is stainless steel corrosion?
First things first, stainless steel corrosion is a misnomer. In all fairness, the name makes sense as the corrosion happens together with stainless steel. And in this blog post, I will only talk about stainless steel corrosion in the conjunction between steel and aluminum. However, corrosion can happen between various metals – not only steel – and the official term for that is galvanic corrosion.
Galvanic corrosion refers to corrosion damage when two or more dissimilar materials are coupled and a corrosive electrolyte is present. Usually, one of these metals is noble (e.g. stainless steel or copper) and is called the cathode. The other metal is un-noble (aluminum) and called the anode. The presence of an electrolyte causes a current flow, which in turn creates corrosion damage. What do I mean by electrolyte? Think about water puddles on a bridge or rain on a façade.
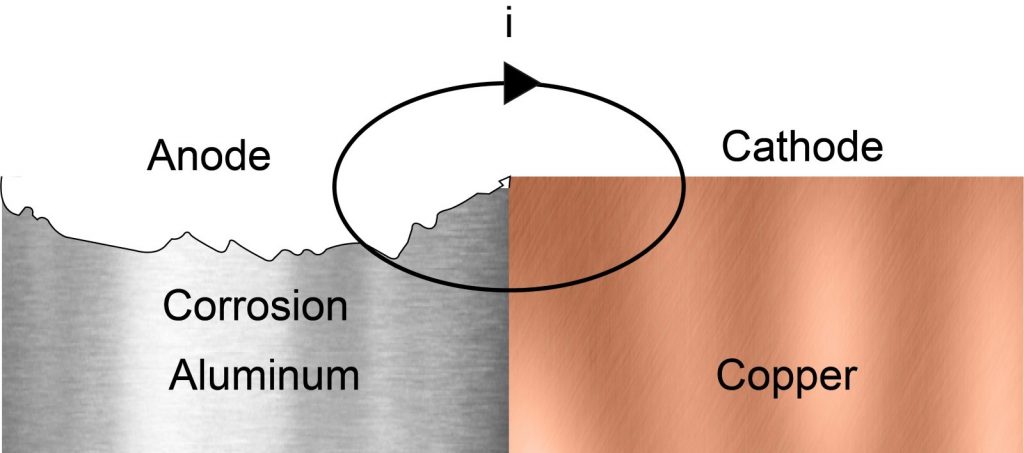
The drawing shows aluminum and copper in contact with each other, same would happen if copper is replaced with stainless steel. Aluminum acts as the anode, which will oxidize, meaning aluminum will corrode. Meanwhile, the cathode (copper or stainless steel) stays intact and helps the current to flow. How fast this corrosion will happen depends on the anode/cathode ratio. A future blog post will go more in-depth about what exactly happens in the galvanic corrosion process – you can follow me on LinkedIn to not miss any new blog posts!
Why does aluminum – stainless steel corrosion happen?
The good news is: the galvanic corrosion of aluminum is usually mild, except in highly conductive media such as slated slush from road de-icing salts, seawater, and other electrolytes. The contact area must be wetted with an aqueous liquid, or humid environment in order to ensure ionic conduction. Otherwise, there will be no possibility of galvanic corrosion.
Even better news: it is unusual to see galvanic corrosion on aluminum in contact with stainless steel (passive). But wait, why am I writing a whole blog post about aluminum – stainless steel corrosion if it is not a common problem?
The reason why you still need to be on the lookout for galvanic corrosion between aluminum and stainless steel is that stainless steel can be found passive or active. Plus, chloride in the environment will also change the corrosion effect on aluminum substantially. Normally the galvanic coupling with stainless steel works very well but when there is even the slightest trace of chloride in the environment galvanic corrosion will take place.
Now, one of the ways to prevent aluminum – stainless steel corrosion is anodizing the aluminum part. However, if the anodic coating is not a intact protecting the surface, it can even make the corrosion worse.

This picture shows a test block made of anodized aluminum. This block has been tested in an accelerated corrosion test. In theory, the anodic coating serves as a protective layer separating the aluminum test block from the stainless steel screws. But when the screws are mounted into the anodized aluminum block without enough care, small cracks in the oxide layer appear. Some of these cracks go down to the un-noble aluminum – leaving us with small anodic areas (aluminum) and a much bigger cathodic area – the stainless steel screw. This is where galvanic corrosion will start, as seen in the picture.
How to prevent aluminum – stainless steel corrosion?
Now to the most important question: How to prevent aluminum – stainless steel corrosion from ever happening? There are four different methods to prevent galvanic corrosion.
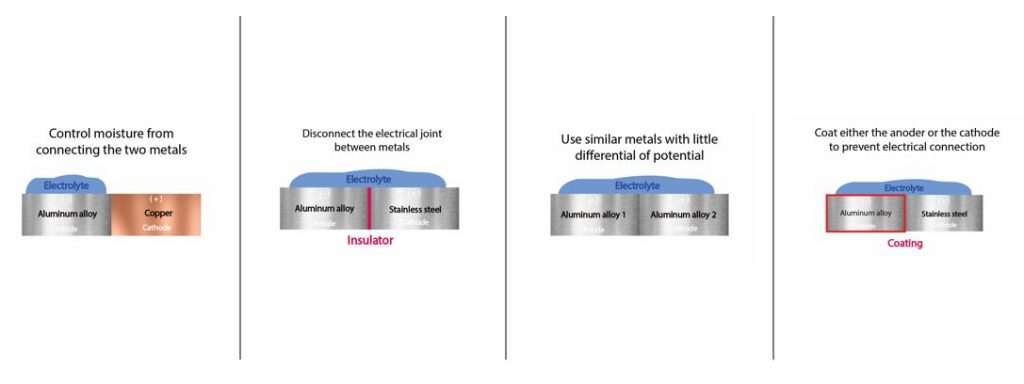
- Disconnect the electrical joint between metals: Use an insulation ring to keep the aluminum and the stainless steel separate from each other.
- Use similar metals: Make sure that you are using passive stainless steel and not cheaper steel in contact with the aluminum.
- Control moisture from connecting the two metals: Make sure that one or both metal parts do not get in contact with chloride and exist in a neutral pH level range.
- Coating: Coat the aluminum in an anodic layer. However, you have to ensure a homogenous high-quality anodized surface. Only then will you prevent galvanic corrosion. Here you can read more about sealing aluminum to prevent stainless steel corrosion.
Depending on your application, anodizing might be the best option to prevent galvanic corrosion. Are you not sure if anodizing is the right choice for you or do you have questions about the specification? Contact me about consultancy or customized workshops on any topic of your choice. I consult both anodizers as well as OEMs, engineers, designers, and anyone else who is working with anodized aluminum parts.
If you liked the blog post, you might also like our blog post about the three most common corrosion types in buildings.
If you are working with anodized parts, we have a practical checklist for you! This checklist helps you to get exactly the anodized parts you want for your product. Simply fill out the form and receive the checklist.